Discovery of electrons
J.J Thomson in 1990 discovered cathode rays for electrons originating for a meeting from the cathode in a gas discharge tube. Electrons are the fundamental particle of all atoms.
Cathode rays travel in a straight line in the presence of electric field these get deflected towards positive electrode. They produce fluorescence when is strike on the wall of discharge tube.
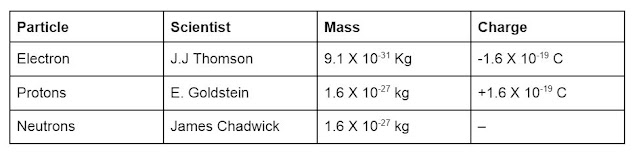
Charge and mass of electron are 1.6 X 10-19 C and 9.1 X 10-31 kg respectively.
Discovery of proton
E. Goldstein in 1886 discovered the presence of new radiation known as Canal rays or anode rays passing through holes or canal of cathode and moving toward cathode in a discharge tube.
Anode Ray consists of positively charged particle known as protons.
Proton have a charge equal in magnitude but opposite in sign to that of electron. Its mass is about 1840 times of as that of electron.
Discovery of neutrons
James Chadwick discovered another subatomic particle called neutrons. They are electrically neutral and are as heavy as protons. They are present in the nucleus of all atoms except hydrogen.
Electron, Proton and Neutron
Thomson model of an atom
Postulates are:
The mass of an atom is assumed to be uniformly distributed throughout atom.
An atom is considered to be a sphere of of uniformly distributed positive charge in which electrons are embedded.
The negative and positive charge balance each other therefore atom as a whole is neutral.
Rutherford Model of an atom
After performing Alpha particle experiment he suggested that:
There is a positively charged highly dense center in an atom called the nucleus, nearly the whole mass of atom resides in it.
The electron revolve around the nucleus in well defined path called Orbit.
Drawbacks of Rutherford Model
Unable to explain the stability of an atom.
Unable to explain the electronic structure of an atom.
Bohr model of an atom
Postulates are:
Only certain special orbits called discrete Orbits or energy levels of electrons are allowed inside the atom.
While revolving in discrete orbits the electrons do not radiate energy.
The orbit are represented by letter K, L, M, N or the number 1, 2, 3, 4.
Bohr and Bury scheme for distribution of electron in different energy level
The maximum number of electron present in an energy level is equal to 2n2, where n is the energy level or Orbit of the shells. Therefore, the maximum number of electrons in different shells are as follows:
First orbit or K-shell = 2 X (1)2 = 2.
Second orbit or L-shell = 2 X (2)2 = 8.
Third orbit or M-shell = 2 X (3)2 = 18.
Valency
It is the combining capacity of an element with the atoms of other elements in order to complete its octet.
Atomic number
It is defined as the number of proton present in the nucleus of an atom. It is also equal to the number of electron in case of neutral atom. It is denoted by Z and written as subscript example 6C.
In neutral atom,
Atomic number = number of protons = number of electrons.
Mass number
It is defined as the number of protons and neutrons in the nucleus. It is denoted by A. example C12.
Mass Number = number of protons + number of neutrons.
Isotopes
They have the same atomic number but different mass number or same number of protons but different number of neutrons. example 1H1 1H2 1H3, etc
Their chemical properties are same due to the same atomic number.
Isobars
They have different atomic number but same mass number. Their physical and chemical properties are different. example 18Ar40 20Ca40, etc.